An article in the BBC caught my attention the other day entitled “Is Komodo dragon blood the key to new antibiotics?” I was excited because I knew of the specialised venom which komodo dragons possess that causes severely low blood pressure by widening blood vessels, thereby inducing shock in prey and also has the effect of prolonging and increasing blood loss. I was hopeful that this venom would be the element of the komodo dragon that was being explored. However, as I began to read the article I was slightly disheartened by even the first bolded line: “Komodo dragon blood contains an important compound which scientists think could offer a new treatment for infected wounds.”
I was disheartened, one – because the article wasn’t in fact about toxins; nonetheless, I was still intrigued, and two – because of the indeterminate terms “important compound” and “scientists think”. In my experience this either means the article is not thoroughly researched and, therefore, not reliable, going to be extremely vague or purely written as a form of “click-bait” to sell more content. I accept that this is the beginning summary of the article and the rest of it was ever so slightly more specific, but I am usually a big fan of BBC and refer to it as one of the more reliable sources of general news but this was not a brilliant article. I also realise that the BBC is designed to inform on all current events and not a specialist in science journalism. Still, antibiotic resistance is (in my opinion at least) one of the biggest issues that will face my generation and it is what this article should be placing emphasis on, but it is barely mentioned twice and only really focuses on MRSA (Methicillin-resistant Staphylococcus aureus).
Anyway, it was not my intention to write BBC news a scathing review (I am actually a big fan of the BBC and particularly like its political reporting). I wanted to talk about the issue of antibiotic resistance and potential solutions, including the komodo dragon route.
Firstly, a smidgen of history. Homo sapiens have always had antibiotics in a way, even if we did not realise it. We exploited the antibiotic properties of plants and created natural medicines from garlic, chamomile, rhubarb, thyme, and hundreds more. Our less natural idea of the origin of antibiotics begins with Fleming discovering penicillin in 1928. And still, the first of the “modern day” antibiotics, which are sulphur containing compounds, arose in 1935 with Prontosil rubrum. Also, it was actually not until 1940 that penicillin was purified, and used as an official antibiotic which saved the lives of many soldiers in the later stages of WWII. At this point people anticipated the defeat of all infectious diseases that had plagued humankind since we had opposable thumbs and probably even before then.
This is ironically where the problem began. Excited by the remarkable healing power of antibiotics medical professionals began the widespread and occasionally unnecessary use of them. Simply, this misuse and overuse of antibiotics has lead to antimicrobial resistance. It is due to the evolution and rapid reproductive abilities of bacteria. Antibiotics kill or inhibit the growth of susceptible bacteria. Sometimes one of the bacteria survives because it has a mutation that gives it the ability to either neutralise the antibiotic, pump the antibiotic out or even change the antibiotic attack site so it cannot affect the function of the bacteria; that one bacterium can then multiply and replace all the bacteria that were killed off. Exposure to antibiotics therefore provides selective pressure, which makes the surviving bacteria more likely to be resistant.
(after uploading this post I found this absolutely brilliant TED talk by Kevin Wu on antibiotic resistance which is worth watching: https://youtu.be/znnp-Ivj2ek )
In addition, another way this resistant trait can be passed on is if bacteria that were once susceptible to an antibiotic gained resistance by acquiring pieces of DNA that code for the resistance from other bacteria. This is called bacterial conjugation and means that bacteria can become resistant to many antimicrobial agents without the need for multiple generations to form because of the transfer of one piece of DNA to all of the organisms.
*side note: mechanism of bacterial conjugation*
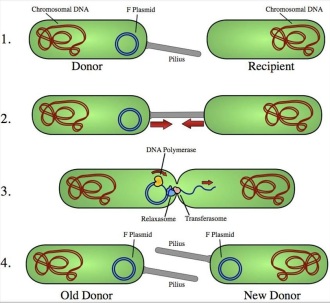
- The donor cell produces a pilus (short, hair-like bacterial cell-surface projections used for adhering to other bacterial cell rather than propelling the bacterium like flagella).
- The pilus attaches to recipient cell and brings the two cells together.
- The mobile plasmid is sliced and a single strand of DNA is then transferred to the recipient cell.
- Both cells synthesize a complementary strand and make a double stranded circular plasmid. They now both possess the DNA that codes for resistance and also produce pili so can be donors
So, the more we use antibiotics the less effective they become and unfortunately we use antibiotics a lot – a lot, a lot – way too much. The problem is becoming increasingly worse for many reasons including:
- Unnecessary prescription of antibiotics for viral infections, such as the common cold and flu.
- Too frequent prescription of “broad-spectrum antibiotics”, in place of a better targeted antibiotics due to lack of precise diagnosis.
- Limited access to medical care and effective treatments leading people to self-medicate and the availability of counterfeit drugs has exacerbated drug resistance developing in this way
- Extensive use in agriculture as growth supplements in livestock. Antibiotics are used primarily to promote growth and to prevent infection as it improves overall health of the animals, producing larger yields and a higher-quality product.
- Low availability of new antibiotics due to economic and regulatory barriers in the pharmaceutical industry.
There is some more detail about this and many supporting statistics in The Antibiotic Resistance Crisis, Part 1: Causes and Threats by C. Lee Ventola, a consultant medical writer in New Jersey (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521/)
Antibiotic resistant infections are such a big issue because they are not only costly, difficult to treat, and potentially deadly, but they can also spread rapidly, affecting entire communities in a short amount of time. In addition, the scariest thing is, if resistance to treatment continues, our interconnected, medically advanced world may revert back to the dark ages of medicine, before today’s “miracle” drugs ever existed and infectious diseases were the leading cause of death with deadly epidemics being common.
But the komodo dragon story, however uninformative, does hint that other solutions for antibiotic resistance are being devised.
I found a variety of different articles from more reputable and appropriate sources (The Science Times, Nature, Science News, American Chemical Society, etc.)
The interest in Komodo dragons began after an observation of how the reptiles rarely become ill despite their diet of rotting flesh and saliva that was thought to be jam-packed with harmful bacteria. (It was believed that komodo dragons used the bacteria in their mouths to cause fatal infections in their prey and wait for the wounded animals to succumb to the infection; however, Prof. Brian Goldstein disproved this by analysing the bacteria in the dragons’ mouths.“The levels of bacteria in the mouth are lower than you’d get for a captive mammalian carnivore, such as a lion or Tasmanian devil,” says Fry. “Komodos are actually remarkably clean animals.” Instead, the animals that were thought to have been killed by the komodo dragon-borne infections were dying from wounds that became infected on contact with faeces infested river water) Despite the misinformed initiating factor to the investigation, the research has reaped some rewards. Komodo dragons would also need antibiotic features to disinfect the wounds they receive from frequent interspecies fighting. There was a spectacular Komodo Dragon fight on Planet Earth II (https://www.youtube.com/watch?v=3Q05CSZAa8U)
Barney Bishop, Monique van Hoek and a team of researchers from the School of Systems Biology at George Mason University hypothesised that the remarkable health of the komodo dragons was due to cationic antimicrobial peptides (CAMPs) and in particular a CAMP found in their blood named VK25.
To find VK25 the researchers used an approach known as bioprospecting. This involves mixing Komodo dragon blood with negatively charged hydrogel particles that capture the peptides, which would have been given a positive charge. 48 potential CAMPs were identified and sequenced using De Novo peptide sequencing mass spectrometry, which is a method of identifying amino acids from peptide fragments using their differing masses. It was discovered that 47 of the 48 CAMPs are derived from histone proteins, which are known to have antimicrobial activities and 8 of these were tested against Pseudomonas aeruginosa and Staphylococcus aureus. Seven of the peptides showed significant potency against both bacteria with an eighth only being effective against P. aeruginosa.
Of these 7 it was VK25 that they found to be the most viable candidate as a treatment for infections as it can also prevent biofilms (robust layer of mucus containing microorganisms that can adhere to surfaces) from forming, which often found in infected wounds.
Van Hoek and team experimented with rearranging some amino acids present in VK25 with the aim of making it more effective. The swapping of two of the amino acids led to the development of synthetic version of the peptide, which they named DRGN-1, which is “stronger in terms of both potency and stability,” says van Hoek.
Following this, the team began to test DRGN-1 on mice with wounds that were infected with two strains of antibiotic-resistant bacteria: Pseudomonas aeruginosa and Staphylococcus aureus.
The synthetic peptide first attacked and destroyed the biofilm of the wounds, before killing the two bacterial strains leading to a reportedly faster wound-healing process.
Van Hoek has said “Synthetic germ-fighter peptides are a new approach to potentially defeat bacteria that have grown resistant to conventional antibiotics. The antimicrobial peptides we’re tapping into represent millions of years of evolution in protecting immune systems from dangerous infections.”
This seems very promising, but is a pretty optimistic view – there are still human clinical trials to be held which will involve many ethical implications such as giving people infections that are potentially deadly with no dependable auxiliary treatment in case the trial drug fails.
Another approach to antibiotic resistance that I have come across involves sea sponges. There are actually two routes leading to sea sponges. One uses antarctic sea sponges Dendrilla membranosa with the compound dubbed “darwinolide” that has antibiotic properties and can destroy biofilms alike VK25. The other uses marine sponges from the Bahamas with the antibacterial compound “dragmacidin G”.
From the Antarctic, the “darwinolide” compound has created excitement because of its chemical structure and how entirely different it is from the antibiotics we use today, such as sulfonamides:
Structure of darwinolide (left) and structure of sulfamethoxazole, a sulfonamide (right)
It is hoped that this compound may provide a new blueprint for future synthetic antibiotics. When unmodified darwinolide was tested against MRSA it killed 98% of cells, which was seen as a success; although, when I read it I only thought of the remaining 2% that are likely to be the strongest of the MRSA and are now left to multiply without competition, which could only make the MRSA problem worse. However, darwinolide could be further explored and artificially enhanced to be more effective.
More information on “darwinolide” can be found here: https://phys.org/news/2016-06-antarctic-sponge-deadly-mrsa-infection.html
The Bahamian sea sponges of the genus Spongosorites that contain the compound “dragmacidin G” have been attributed with having antibacterial, antiviral, antifungal, antiplasmodial (fights parasites), cytotoxic and anti-inflammatory properties. FAU Harbor Branch’s drug discovery program has been investigating Spongosorites looking for treatments for infectious diseases such as TB and malaria (as well as pancreatic cancer (dragmacidin G is thought to inhibit pancreatic cancer cell membrane formation), other forms of cancer, neurodegenerative disease and inflammation). Dragmacidin G has only undergone preliminary tests but the results are promising and further research is taking place. Dr Amy Wright, who directs FAU’s Harbor Branch’s drug discovery program, and a team are the ones conducting the research and are also exploring the disparate structure of dragmacidin G as a potential new template for antibiotics and have already began modifications:
Structure of Dragmacidin G (left) and its pyrimidine derivative (right).
More information can be found here: http://www.mdpi.com/1660-3397/15/1/16
There is actually a lot of different research surrounding antibiotic resistance. Whilst gathering information for this I also found “lysibodies”, molecules created by Rockefeller University inspired by viruses that can kill bacteria. They are hybrids of human antibodies and lysins – molecules that bind to carbohydrates on cell walls. They kill bacteria by latching on to carbohydrates on the bacterial cell walls and the antibody part of the lysibody then attacks. The report by Vincent A Fischetti can be found here: https://bmcoralhealth.biomedcentral.com/articles/10.1186/1472-6831-6-S1-S16

